Feature
· After two full years of therapy with Cosentyx 300 mg, almost 9 out of 10 psoriasis patients sustained their PASI 75 response1
· New data at AAD shows 7 out of 10 psoriasis patients, who were PASI 75 responders at 52 Weeks, had almost clear to clear skin (PASI 90 to PASI 100) after two years of Cosentyx 300 mg treatment1
· Cosentyx is the first and only IL-17A inhibitor approved in Europe, the US, Japan, Canada and Switzerland for moderate-to-severe plaque psoriasis2-4
Basel, March 21, 2015 – Novartis today announced new two-year results demonstrating strong and sustained efficacy with Cosentyx™ (secukinumab) with a favorable safety profile for the treatment of psoriasis patients1. The data comes from the extension study of the pivotal Phase III FIXTURE and ERASURE trials. Results were presented for the first time in a late-breaking session at the 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco, USA. Cosentyx is the first and only interleukin-17A (IL-17A) inhibitor approved to treat adult moderate-to-severe plaque psoriasis patients.
In this extension of the FIXTURE and ERASURE studies, 995 patients who achieved Psoriasis Area Severity Index (PASI) 75 response after a year of therapy (Week 52) received either Cosentyx 300 mg, Cosentyx 150 mg or placebo for an additional year (Week 104)1. After two full years of therapy, 7 out of 10 (71%) patients treated with Cosentyx 300 mg had clear or almost clear skin (PASI 90); 4 out of 10 (44%) had clear skin (PASI 100) and almost 9 out of 10 (88%) patients maintained their PASI 75 response1. PASI assesses treatment efficacy by measuring the reduction in redness, scaling and thickness of psoriatic plaques and the extent of involvement in each region of the body5,6.
“We are pleased to share new long term data showing how the sustained efficacy and favorable safety profile of Cosentyx helps psoriasis patients maintain clear or almost clear skin over two years of treatment,” said Vasant Narasimhan, Global Head of Development, Novartis Pharmaceuticals. “Psoriasis is a chronic condition causing itching, scaling and pain; patients need therapies that provide rapid relief and clear skin over a long period of time.”
In the study, 70% of patients who initially received placebo and were switched to receive Cosentyx 300 mg after losing treatment response, were able to achieve PASI 90 within 12 weeks of starting Cosentyx treatment1. The safety profile of Cosentyx was favorable and consistent with previously reported Phase III clinical trials. No new or unexpected safety findings were identified during the two year extension1. The most common adverse were nasopharyngitis, upper respiratory tract infection, hypertension, headache and arthralgia.
About the A2302E1 Extension Study (Cosentyx Extension Study to the FIXTURE and ERASURE studies)
A2302E1 is a multicenter, double-blind, randomized withdrawal extension study to the FIXTURE and ERASURE pivotal Phase III studies. The extension study was conducted to collect long term efficacy, safety and tolerability data on Cosentyx in patients who achieved a PASI 75 response to Cosentyx at Week 52 of the FIXTURE and ERASURE core studies in moderate-to-severe plaque psoriasis.
Patients who had been receiving Cosentyx 300 mg or 150 mg during the maintenance period of the core studies, and who exhibited a PASI 75 response at Week 52 of the core studies, were randomized to continue the same Cosentyx dose or receive placebo1. Patients who exhibited partial response (PASI 50 to <PASI 75 response) from baseline at Week 52 of the core studies were also eligible to enter A2302E, but did not enter the randomized withdrawal extension study1. Partial responders instead continued the same treatment dose (Cosentyx 300 mg or 150 mg) that they received at the time of completing the maintenance period (Week 52) in the core studies. Non-responders (patients who did not achieve at least a PASI 50 response at Week 52 of the core study) were not eligible to enter any part of this extension study1.
About the FIXTURE and ERASURE studies
FIXTURE (the Full year Investigative eXamination of secukinumab vs. eTanercept Using 2 dosing Regimens to determine Efficacy in psoriasis) and ERASURE (Efficacy of Response And Safety of two fixed secUkinumab REgimens in psoriasis) are part of one of the largest Phase III program in moderate-to-severe plaque psoriasis completed to date, which involved more than 3,300 patients in over 35 countries7.
FIXTURE and ERASURE assessed the efficacy, safety and tolerability of induction period (at Week 12) and maintenance therapy (at Week 52) with subcutaneous Cosentyx 300 mg or 150 mg in patients with moderate-to-severe plaque psoriasis7. Both studies were multicenter, randomized, double-blind, placebo-controlled (FIXTURE: also active controlled), parallel-group Phase III trials involving 1,306 patients and 738 patients with moderate-to-severe plaque psoriasis, respectively7. Each study consisted of a 1-to-4-week screening period, a 12-week induction period, a 40-week maintenance period and an 8-week follow-up period7. FIXTURE was the first full-year blinded, direct comparison of biologic therapies for psoriasis in a Phase III study7.
The co-primary endpoints in both studies, PASI 75 response and Investigator's Global Assessment (IGA mod 2011) 0/1 response at Week 12, were used to demonstrate superiority of Cosentyx vs. placebo (p<0.001 for all comparisons)7. Cosentyx 300 mg demonstrated significant improvements in PASI 75 at Week 12 (77.1% vs. 44.0% for Enbrel®* vs. 4.9% for placebo in FIXTURE and 81.6% vs. 4.5% for placebo in ERASURE)7.
About Cosentyx (secukinumab) and interleukin-17A (IL-17A)
Cosentyx is a human monoclonal antibody that selectively neutralizes interleukin-17A (IL-17A)8,9. IL-17A is found in high concentrations in skin affected by psoriasis and is a preferred target for investigational therapies8-13. Cosentyx works by inhibiting the action of IL-17A, a protein found in high concentrations in skin affected by the disease8-13. In the Phase III program, Cosentyx demonstrated a favorable safety profile, with similar incidence and severity of adverse events between Cosentyx treatment arms (300 mg and 150 mg)1,7.
In January 2015, Cosentyx (at a dose of 300 mg) became the first and only IL-17A inhibitor approved in Europe as a first-line systemic treatment of moderate-to-severe plaque psoriasis in adult patients, and in the US as a treatment for moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy (light therapy). In addition to the EU and the US, Cosentyx has been approved in Switzerland, Chile, Australia, Canada and Singapore for the treatment of moderate-to-severe plaque psoriasis and in Japan for the treatment of moderate-to-severe plaque psoriasis and active psoriatic arthritis (PsA).
Cosentyx is also in Phase III development for PsA and ankylosing spondylitis (AS); global regulatory applications are planned for 2015.
About psoriasis
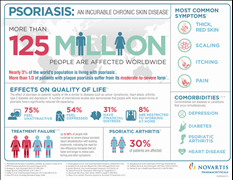
Psoriasis is a chronic immune-mediated disease characterized by thick and extensive skin lesions, called plaques, known to cause itching, scaling and pain; it is associated with significant impairment of physical and psychological quality of life14-16. Psoriasis affects up to 3% of the world’s population, or more than 125 million people17.
This common and distressing condition is not simply a cosmetic problem – even people with very mild symptoms are affected everyday2. According to an analysis of surveys conducted of 5,600 patients by the National Psoriasis Foundation (NPF) between 2003 and 2011, 52% of patients with mild, moderate and severe psoriasis were dissatisfied with their disease management18. Of the patients surveyed, some were receiving no treatment (9.4-49.2%) or were undertreated (10.2-55.5%)18.