Novartis’ Cosentyx is first and only IL-17A inhibitor to potentially modify the course of psoriasis
- New data suggests that disease modification with Cosentyx may be achievable for around 20% of patients following one year of treatment1
- Patients with longer disease duration before treatment with Cosentyx were more likely to relapse, highlighting the potential importance of early treatment with Cosentyx1
- To further investigate disease modification, Novartis has initiated the STEPIn trial, evaluating Cosentyx in patients with early onset of moderate-to-severe psoriasis2
Basel, March 21, 2017 – Novartis today announced new data suggesting, for the first time, that Cosentyx® (secukinumab) may modify the course of moderate-to-severe psoriasis leading to long-term, treatment-free skin clearance1. Cosentyx is the first and only IL-17A inhibitor to have reported this potential of disease modification. These data were presented at the 13th Annual Maui Derm for Dermatologists 2017, Maui, Hawaii at which Novartis presented 14 abstracts.
Following one year of treatment with Cosentyx, patients were randomized to either continuous treatment or treatment cessation until relapse. Patients with continuous treatment maintained their high level of response. Among the patients that discontinued treatment, 21% of psoriasis patients maintained skin clearance for up to one year without treatment and 10% maintained skin clearance after two years without treatment1. Patients with longer disease duration were more likely to relapse, suggesting that early intervention increases the chance of remaining relapse free1.
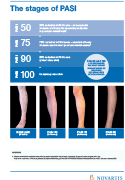
Previous data has shown that Cosentyx, a fully human, specific inhibitor of the IL-17A cytokine, delivers long-lasting clear or almost clear skin (PASI 90 to PASI 100) in up to 80% of patients out to four years3,4.
"These results suggest that Cosentyx may go beyond simply treating symptoms and could actually modify the course of psoriasis, and highlights the need for further investigation into early intervention," said Vas Narasimhan, Global Head, Drug Development and Chief Medical Officer, Novartis. "Being able to change the course of disease is the ultimate goal of treatment, which is why we are investing in the STEPIn trial to further understand the disease modifying ability of Cosentyx in psoriasis."
This is the first robust long-term data on psoriasis following treatment discontinuation. These data (from extension study A2302E1) show low scores on the Psoriasis Area Severity Index (PASI) were maintained after treatment discontinuation following one year on Cosentyx (PASI score of 2.9 after 1 year and 1.7 after 2 years off-drug, vs. 20.5 and 19.2 at Baseline)1. Additionally, of the 120 patients who were PASI 75 responders and switched to placebo at one year, 21% remained relapse-free after one year and 10% were relapse-free after two years off-treatment1. Patients who had a longer disease duration before Cosentyx treatment were more likely to relapse, highlighting the potential importance of early treatment1. To further investigate the disease modification potential of Cosentyx, Novartis has initiated the STEPIn trial to assess early intervention with Cosentyx in new-onset disease. The ambition is to identify a novel strategy of treating patients with new-onset moderate-to-severe psoriasis, by providing evidence to inform the use of early treatment2.
Cosentyx is the only IL-17A inhibitor approved in psoriasis, psoriatic arthritis and ankylosing spondylitis. 80,000 patients have been treated worldwide in the post-marketing setting5.
About Cosentyx and interleukin-17A (IL-17A)
Launched in January 2015, Cosentyx is a targeted treatment that specifically inhibits the IL-17A cytokine. Cosentyx delivers long-lasting clear skin, with proven sustainability, safety out to four years and convenient once-monthly dosing in a patient-friendly auto injector6.
Cosentyx is approved in more than 75 countries for the treatment of moderate-to-severe plaque psoriasis, which includes the European Union countries, Japan, Switzerland, Australia, the US and Canada. In Europe, Cosentyx is approved for the first-line systemic treatment of moderate-to-severe plaque psoriasis in adult patients7. In the US, Cosentyx is approved as a treatment for moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy (light therapy)8.
In addition, Cosentyx is the first IL-17A inhibitor approved in more than 65 countries for the treatment of active AS and PsA, which includes the European Union countries and the US. Cosentyx is also approved for the treatment of PsA and pustular psoriasis in Japan5.
About A2302E1
A2302E1 is an extension of pivotal phase 3 studies ERASURE and FIXTURE made up of a double-blind, placebo-controlled study of 120 psoriasis patients. After one year of Cosentyx treatment, patients who achieved a PASI 75 response were randomized to receive either Cosentyx 300mg or placebo. During the treatment withdrawal concomitant psoriasis medication was prohibited. Upon relapse placebo patients were retreated with Cosentyx.
About STEPIn2
A randomized, multicenter study to evaluate the effect of secukinumab 300 mg s.c. administered during 52 weeks to patients suffering from new-onset moderate-to-severe plaque psoriasis as early intervention compared to standard of care treatment with narrow-band UVB. STEPIn aims to demonstrate the benefit of early secukinumab treatment with the ultimate goal of altering the natural course psoriasis with a reduced disease burden and need for treatment.
About psoriasis
Psoriasis is a common, non-contagious, autoimmune disease that affects more than 125 million people worldwide9. Plaque psoriasis is the most common form of the disease and appears as raised, red patches covered with a silvery white buildup of dead skin cells.
Psoriasis is not simply a cosmetic problem, but a persistent, chronic (long-lasting), and sometimes distressing disease, which can affect even the smallest aspects of people's lives on a daily basis. Up to 30% of patients with psoriasis have, or will develop, PsA10. PsA is a condition in which the joints are also affected, causing debilitating symptoms including pain, stiffness and irreversible joint damage10,11. Psoriasis is also associated with other serious health conditions, such as diabetes, heart disease and depression8.
Disclaimer
The foregoing release contains forward-looking statements that can be identified by words such as "potentially," "suggests," "may," "potential," "investigate," "initiated," "suggesting," "suggest," "could," "investigation," "goal," "investing," "ambition," "launched," "aims," or similar terms, or by express or implied discussions regarding potential new indications or labeling for Cosentyx, or regarding potential future revenues from Cosentyx. You should not place undue reliance on these statements. Such forward-looking statements are based on the current beliefs and expectations of management regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that Cosentyx will be submitted or approved for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that Cosentyx will be commercially successful in the future. In particular, management's expectations regarding Cosentyx could be affected by, among other things, the uncertainties inherent in research and development, including clinical trial results and additional analysis of existing clinical data; regulatory actions or delays or government regulation generally; the company's ability to obtain or maintain proprietary intellectual property protection; general economic and industry conditions; global trends toward health care cost containment, including ongoing pricing pressures; safety, quality or manufacturing issues, and other risks and factors referred to in Novartis AG's current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements contained in this press release as a result of new information, future events or otherwise.