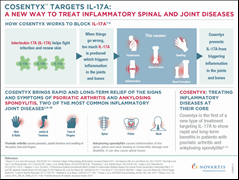
Novartis receives two new FDA approvals for Cosentyx to treat patients with ankylosing spondylitis and psoriatic arthritis in the US
· Cosentyx is the first and only interleukin-17A (IL-17A) inhibitor approved for adult patients with ankylosing spondylitis (AS) and psoriatic arthritis (PsA)
· FDA approval for Cosentyx is based on efficacy and safety outcomes shown across four Phase III studies, including over 1,500 patients with either AS or PsA1
· In studies, Cosentyx met the primary endpoints showing statistically significant improvements versus placebo in the signs and symptoms of AS and PsA1
Basel, January 15, 2016 – Novartis announced today that the US Food and Drug Administration (FDA) has approved Cosentyx® (secukinumab) for the treatment of two new indications — adults with active ankylosing spondylitis (AS) and active psoriatic arthritis (PsA). AS and PsA are both life-long, painful and debilitating inflammatory diseases that affect the joints and/or spine. If not treated effectively, both conditions can lead to irreversible joint and/or spinal bone damage caused by years of inflammation2,3.
Cosentyx is the first in a new class of medicines called interleukin-17A (IL-17A) inhibitors to treat both AS and PsA. The two new indications follow the earlier FDA approval for Cosentyx in January 2015 to treat adult patients with moderate-to-severe plaque psoriasis, and European approval for AS and PsA in November 2015.
“These new approvals are a potential turning point for people living with ankylosing spondylitis and psoriatic arthritis in the US, as Cosentyx provides a novel and targeted way of inhibiting the inflammatory process of these two conditions,” said David Epstein, Division Head, Novartis Pharmaceuticals. “The results from our studies have shown that the majority of patients treated with Cosentyx have a significant reduction in their signs and symptoms of ankylosing spondylitis and psoriatic arthritis, and show major improvements in their ability to undertake everyday activities.”
In the US, it is estimated that up to 0.5% of the population have AS, and up to 1% live with PsA4,5. If not treated effectively, these conditions can lead to irreversible damage to the spine and joints, causing life-long pain and disability that can have a negative impact on even simple tasks in life2,3. There is an urgent unmet need for new medicines for these conditions. Currently many patients are dissatisfied with their treatments, and up to 40% do not respond sufficiently to anti-tumor necrosis factor-a (anti-TNFs) therapy6.
The approvals are based on the efficacy and safety outcomes from four placebo-controlled Phase III studies, which included over 1,500 adult patients with AS or PsA that were biologic treatment naïve or had an inadequate response / were intolerant to anti-TNFs1. In the studies, Cosentyx met the primary endpoints achieving statistically significant improvements versus placebo in the signs and symptoms of AS and PsA, as measured by at least a 20% improvement in the Assessment of Spondyloarthritis International Society criteria (ASAS 20*) at Week 16, and a 20% reduction in the American College of Rheumatology (ACR 20) response criteria at Week 24, respectively1. ASAS 20 and ACR 20 are standard tools used to assess clinical improvement in AS and PsA.
More than 9,600 patients have been treated with Cosentyx in clinical trials across multiple indications, and over 15,000 patients with psoriasis have already been treated in the post-marketing setting7. The safety profile of Cosentyx was shown to be consistent with that seen in clinical trials across multiple indications7,8-10.
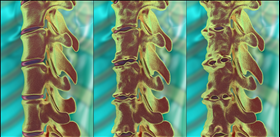
About ankylosing spondylitis (AS)
· Ankylosing spondylitis (AS) is painful and often progressively debilitating, caused by spine inflammation that can result in irreversible damage11.
· Up to 70% of patients who go on to develop severe AS will form spinal fusions (where the bones grow together) over 10 to 15 years, which significantly reduces mobility12.
· People aged 25 or older, particularly males, are affected most often13.
· Family members of those with AS are at higher risk13.
· Approximately 20-40% of patients do not respond well to standard of care biologic medicines, and there are few therapeutic options available to those people6.
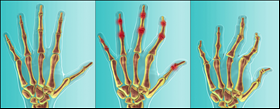
About psoriatic arthritis (PsA)
· Psoriatic arthritis (PsA) is an inflammatory condition of the joints and is often associated with a scaly skin condition called psoriasis3.
· Symptoms include joint pain and stiffness, skin and nail psoriasis, swollen toes and fingers and persistent painful enthesitis (inflammation of the sites where tendons or ligaments insert into the bone)3.
· Up to 40% of people can suffer from joint destruction and permanent physical deformity3,14.
· Up to 15% of people with psoriasis may have undiagnosed PsA15.
· As many as 30% of patients with psoriasis will have PsA16.
· New medicines are needed as many patients do not respond to, or tolerate, current therapies and approximately 45% of PsA patients are dissatisfied with treatments17.
About Cosentyx AS and PsA clinical trial programs
Pivotal Phase III studies in the Cosentyx clinical trial program, that provided key data for the submission, were MEASURE 1 and MEASURE 2 involving 590 patients with AS, and FUTURE 1 and FUTURE 2 including 1,003 patients with PsA. Data from these pivotal trials were published in the New England Journal of Medicine and The Lancet. These are multi-center, randomized, placebo-controlled studies designed to evaluate the efficacy and safety of Cosentyx in AS and PsA. Additional follow-up of patients from these trials is still ongoing8,9,18. Novartis continues to investigate Cosentyx for its potential role in preventing radiographic progression of spinal and joint structural damage in AS and PsA patients respectively, as shown by x-ray.
About Cosentyx and interleukin-17A (IL-17A)
Cosentyx is a fully human monoclonal antibody that selectively neutralizes circulating IL-17A19. Research suggests that IL-17A may play an important role in driving the body’s immune response in psoriasis, PsA and AS20.
Cosentyx is the first IL-17A inhibitor with positive Phase III results for the treatment of PsA and AS8,9,18, and is now approved in Europe and the US for these conditions.Cosentyx is approved for the treatment of AS and PsA in Ecuador and Bangladesh, and for the treatment of PsA in Japan.
In addition, over 50 countries have also approved Cosentyx for the treatment of moderate-to-severe plaque psoriasis which includes the European Union countries, Japan, Switzerland, Australia, the US and Canada. In Europe, Cosentyx is the only first-line biologic approved for the systemic treatment of moderate-to-severe plaque psoriasis in adult patients. In the US, Cosentyx is also approved as a treatment for moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy (light therapy).
Disclaimer
The foregoing release contains forward-looking statements that can be identified by words such as “potential,” “can,” “may,” “will,” “suggests,” or similar terms, or by express or implied discussions regarding potential new indications or labeling for Cosentyx, or regarding potential future revenues from Cosentyx. You should not place undue reliance on these statements. Such forward-looking statements are based on the current beliefs and expectations of management regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that Cosentyx will be submitted or approved for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that Cosentyx will be commercially successful in the future. In particular, management’s expectations regarding Cosentyx could be affected by, among other things, the uncertainties inherent in research and development, including unexpected clinical trial results and additional analysis of existing clinical data; unexpected regulatory actions or delays or government regulation generally; the company’s ability to obtain or maintain proprietary intellectual property protection; general economic and industry conditions; global trends toward health care cost containment, including ongoing pricing pressures; unexpected safety issues; unexpected manufacturing or quality issues, and other risks and factors referred to in Novartis AG’s current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements contained in this press release as a result of new information, future events or otherwise.
*ASAS 20 is improvement of ≥20% and ≥1 unit on a 10-unit scale in at least three of the four core ASAS domains, with no worsening of ≥20% and ≥1 unit in the fourth.