Novartis late-breaking data show Cosentyx delivers high and long-lasting skin clearance over 4 years for psoriasis patients
· Cosentyx® delivers long-lasting clear or almost clear skin (PASI 90 to PASI 100) in the vast majority of patients and continues to show a favorable safety profile over 4 years1
· New data show that with Cosentyx almost 100% of PASI 90 and PASI 100 response rates are maintained from Year 1 to Year 41
· Cosentyx significantly superior to Stelara® in delivering long-lasting skin clearance in psoriasis over 52 weeks, confirms new JAAD publication2
Basel, October 01, 2016 – Novartis announced today new data showing Cosentyx® (secukinumab) delivers high and long-lasting skin clearance in patients with moderate-to-severe plaque psoriasis out to 4 years of treatment1. These late-breaking data were presented for the first time at the 25th European Academy of Dermatology and Venereology (EADV) Congress in Vienna, Austria.
“These impressive results show that Cosentyx keeps working year-on-year, maintaining high levels of skin clearance with a favorable safety profile,” said Vasant Narasimhan, Global Head, Drug Development and Chief Medical Officer, Novartis. “Psoriasis patients need therapies they can use over long periods of time without loss of efficacy and we are pleased that Cosentyx is proving a sustainable choice for patients.”
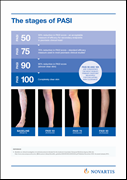
The aim of psoriasis treatment is clear skin, and the Psoriasis Area Severity Index (PASI) 90 response is considered an important measure of treatment success3-6. Clear or almost clear skin (PASI 90) was achieved by 68.5% of patients at Year 1 and this high rate was maintained to Year 4 (66.4%)1. In addition, 43.8% of psoriasis patients achieved completely clear skin (PASI 100) at Year 1 and this rate (43.5%) was maintained to Year 4. The average improvement of psoriasis as measured by the PASI score was maintained at over 90% after 4 years of treatment*. The standard goal of treatment, PASI 75 response, was achieved by 88.5% of patients at Year 4. In this long-term study, Cosentyx continues to have a favorable safety profile, which was consistent with that demonstrated in previous Phase III studies.
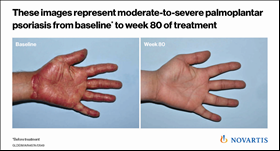
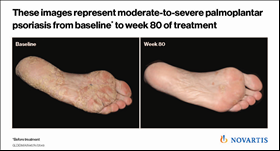
Also presented at EADV were results demonstrating the longer-term efficacy (1.5 years) of Cosentyx in treating psoriasis of the hands and feet (palmoplantar), which are considered difficult areas to treat on the body7. Approximately 60% of patients achieved clear or almost clear palms and soles with Cosentyx, which continued to improve over 1.5 years7. This demonstrates the strength of Cosentyx as an important treatment option for patients with psoriasis on these parts of their body that are crucial for everyday function. These patients are known to suffer greater disability and discomfort than those with psoriasis on other areas8.
Newly published data also show Cosentyx delivers superior, long-lasting skin clearance versus Stelara®† (ustekinumab) for up to 1 year in patients with moderate-to-severe psoriasis: 76% for Cosentyx vs. 61% for Stelara (P<0.0001) at 52 weeks2. Cosentyx has now shown superior and sustained results versus both Stelara and Enbrel®‡, two widely used biologic treatments2,9. This head-to-head CLEAR study was published in advance of the EADV congress in the Journal of the American Academy of Dermatology (JAAD).
About Cosentyx and interleukin-17A (IL-17A)
Cosentyx is a fully human monoclonal antibody that selectively neutralizes IL-17A. Research suggests that IL-17A may play an important role in driving the body’s immune response in psoriasis, ankylosing spondylitis (AS) and psoriatic arthritis (PsA)10,11.
Cosentyx is approved in more than 65 countries for the treatment of moderate-to-severe plaque psoriasis which includes the European Union countries, Japan, Switzerland, Australia, the US and Canada. In Europe, Cosentyx is approved for the first-line systemic treatment of moderate-to-severe plaque psoriasis in adult patients12. In the US, Cosentyx is approved as a treatment for moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy (light therapy)13.
In addition, Cosentyx is the first IL-17A inhibitor approved in more than 50 countries for the treatment of active AS and PsA, which includes the European Union countries and the US. Cosentyx is also approved for the treatment of PsA and pustular psoriasis in Japan.
More than 10,000 patients have been treated with Cosentyx in clinical trial settings across multiple indications, and over 50,000 patients have been treated in the post-marketing setting14.
About the 4 year Cosentyx efficacy study (A2304E1)1
A2304E1 is a multicenter, double-blind and open-label, 4 year extension to the pivotal Phase III SCULPTURE study. In SCULPTURE, PASI 75 responders at Week 12 were randomized to double-blind maintenance treatment of Cosentyx 300 mg or 150 mg, given either at a 4-week fixed-interval regimen or in a retreatment-as-needed regimen. This same treatment regimen was applied for the 642 patients who completed the 52 weeks of treatment and then continued into the extension.
The primary objective of this extension study was to assess the long-term safety and tolerability of Cosentyx in patients with moderate-to-severe plaque psoriasis. Efficacy measures included proportion of patients achieving PASI 75, PASI 90 and PASI 100.
About the CLEAR study
CLEAR (Comparison to assess Long-term Efficacy, sAfety and toleRability of secukinumab vs. ustekinumab) is a multi-center, double-blind, parallel-group study of Cosentyx (n=335) versus Stelara (n=336) to compare efficacy, safety, and tolerability in adults with moderate-to-severe plaque psoriasis. Patients were randomized to receive either Cosentyx (300 mg) by subcutaneous injection at Baseline, Weeks 1, 2, and 3, then every 4 weeks from Week 4, or Stelara (dosing per package label). Cosentyx achieved the primary objective of superior PASI 90 response at Week 16. The 52 week PASI 90 response is a secondary objective in this study. PASI 100 and PROs (including DLQI responses) at 52 weeks are exploratory endpoints2.
About psoriasis
Psoriasis is a common, non-contagious, autoimmune disease that affects up to 3% of the world’s population15. Plaque psoriasis is the most common form of the disease and appears as raised, red patches covered with a silvery white buildup of dead skin cells. Palmoplantar psoriasis, psoriasis involvement of the palms and soles, occurs in up to 40% of plaque psoriasis patients16.
Psoriasis is not simply a cosmetic problem, but a persistent, chronic (long-lasting), and sometimes distressing disease, which can affect even the smallest aspects of people’s lives on a daily basis. Up to 30% of patients with psoriasis have, or will develop, PsA17. PsA is a condition in which the joints are also affected, causing debilitating symptoms including pain, stiffness and irreversible joint damage17,18. Psoriasis is also associated with other serious health conditions, such as diabetes, heart disease and depression17.
Disclaimer
The foregoing release contains forward-looking statements that can be identified by words such as “continues,” “aim,” “goal,” suggests,” “will,” or similar terms, or by express or implied discussions regarding potential new indications or labeling for Cosentyx, or regarding potential future revenues from Cosentyx. You should not place undue reliance on these statements. Such forward-looking statements are based on the current beliefs and expectations of management regarding future events, and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. There can be no guarantee that Cosentyx will be submitted or approved for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that Cosentyx will receive additional regulatory approvals or be commercially successful in the future. In particular, management’s expectations regarding Cosentyx could be affected by, among other things, the uncertainties inherent in research and development, including unexpected clinical trial results and additional analysis of existing clinical data; unexpected regulatory actions or delays or government regulation generally; the company’s ability to obtain or maintain proprietary intellectual property protection; general economic and industry conditions; global trends toward health care cost containment, including ongoing pricing pressures; unexpected safety, quality or manufacturing issues, and other risks and factors referred to in Novartis AG’s current Form 20-F on file with the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements contained in this press release as a result of new information, future events or otherwise.